Summary
Chronic liver diseases, including cirrhosis and liver failure, remain formidable challenges due to their complex progression and limited therapeutic options. Mesenchymal stem cell (MSC) therapy has emerged as a game-changing approach, leveraging its potent immunomodulatory, anti-fibrotic, and regenerative capabilities, along with the ability to transdifferentiate into hepatocytes. This review delves into the latest advances in MSC-based treatments for chronic and end-stage liver diseases, as highlighted in current clinical trials. MSCs derived from bone marrow and umbilical cord have shown remarkable promise in reversing liver damage, improving liver function, and providing hope for patients who do not respond to conventional therapies. When administered through hepatic, portal, or peripheral veins, MSCs have significantly improved liver histology, reduced fibrosis, and restored functional capacity. Furthermore, MSC-derived materials, such as extracellular vesicles and exosomes, are emerging as cutting-edge tools for treating liver failure and mitigating post-transplant complications. While autologous MSC-derived hepatocytes hold promise for non-fatal cirrhosis, allogeneic MSCs are being applied in more severe conditions, including liver failure and transplantation cases. Despite these promising early outcomes, larger trials and long-term studies are essential to fully harness MSCs as a transformative, off-the-shelf alternative to liver transplantation, heralding a new era in regenerative liver therapies.
Introduction
Liver disease accounts for two million deaths annually, representing 4% of all fatalities worldwide (1 in every 25 deaths) [1]. Hepatic diseases, including specific conditions, cirrhosis, liver failure, and transplantation complications, present significant challenges due to their complex pathogenesis and the irreversible damage that occurs before symptoms emerge [2]. Specific conditions, such as autoimmune hepatitis, Wilson’s disease, and biliary atresia, involve persistent inflammation and fibrosis, leading to progressive liver dysfunction. Cirrhosis is marked by extensive fibrosis and disruption of normal liver architecture, often progressing unnoticed until severe damage occurs. Liver failure, whether acute or chronic, represents the terminal stage of severe liver disease and is life-threatening. Currently, biliary atresia is treated surgically (Kasai procedure) or with transplant [3]. Wilson’s disease is treated with chelation and dietary modifications [4]; autoimmune hepatitis is treated with immunosuppressants [5]. Liver cirrhosis management includes lifestyle modifications, with transplantation for severe cases [6]. Liver failure requires urgent treatment, potentially including transplantation [7]. Liver transplantation involves risks like rejection, infection, and disease recurrence, requiring long-term immunosuppressive therapy [8, 9]. Mesenchymal stem cell (MSC) therapy has emerged as a promising treatment for these liver diseases, with immunomodulatory, anti-fibrotic, and regenerative properties. Clinical trials have explored the therapeutic potential of MSCs for various liver diseases, offering new therapeutic avenues for conditions that are currently difficult to treat [10,11,12].
Challenges of hepatic diseases
Specific chronic liver disease
Hepatic diseases encompass a broad spectrum of disorders, each characterized by unique mechanisms, progression patterns, and distinct pathogenesis. Understanding the complexity of these conditions is crucial, as the liver is a vital organ with multifaceted roles, including metabolism, detoxification, and immune regulation [13]. Autoimmune hepatitis is characterized by aberrant immune responses against hepatocytes, leading to chronic inflammation, fibrosis, and eventually cirrhosis if untreated [14]. Wilson’s disease, on the other hand, is a genetic disorder caused by mutations in the ATP7B gene, leading to impaired copper metabolism [15, 16]. This results in copper accumulation in the liver and other organs, causing oxidative stress, cell damage, and liver function impairment. Biliary atresia is a developmental disorder of the biliary system where the bile ducts are abnormally narrowed or absent, leading to neonatal cholestasis, progressive liver injury, and cirrhosis in infancy [17, 18].
End-stage liver disease (liver cirrhosis)
Cirrhosis represents the end stage of many chronic liver diseases and is characterized by extensive fibrosis and the formation of regenerative nodules, leading to the disruption of normal liver architecture [19, 20]. The progression to cirrhosis is marked by the activation of hepatic stellate cells, which are the primary source of extracellular matrix components in the liver [21]. Persistent liver injury, as seen in chronic hepatitis or alcohol abuse, leads to the continuous activation of these cells, resulting in excessive fibrosis. As cirrhosis advances, liver function deteriorates and resistance in the portal venous system increases, leading to severe complications such as coagulopathy, portal hypertension, ascites, and hepatic encephalopathy. The disease typically progresses slowly over months to years, and its insidious nature complicates early diagnosis and treatment.
Liver failure (liver decompensation)
Liver failure is a severe and life-threatening liver condition presenting with sudden or progressive loss of liver function [22, 23]. It can be the result of acute causes such as drug-induced liver injury, viral hepatitis, or ischemia, or it can occur as a consequence of the progression of chronic liver disease to decompensation. The development of liver failure involves extensive liver damage, significant hepatocyte necrosis, and inadequate liver function to meet physical needs, ultimately leading to multi-system dysfunction. This condition often requires urgent medical intervention, including liver transplantation.
Transplantation complications
Transplantation complications present another set of challenges in managing hepatic diseases [24, 25]. Despite being a life-saving procedure, liver transplantation is associated with an array of risks, including organ rejection, infection, and recurrence of the original liver disease. The host immune reaction to the grafted liver can lead to acute or chronic rejection, requiring long-term immunosuppressive therapy and assessment. Moreover, disease recurrence after liver transplantation including HBV/HCV re-infection, malignancy, or autoimmune hepatitis further complicates the prognosis [26].
The nature of the liver and its non-specific symptoms often make it difficult to promptly detect and manage liver problems. The remarkable regeneration capability of the liver has both pros and cons, as it can mask underlying pathology and produce irrevocable damage. Despite advances in medical science, liver disease remains a critical challenge due to its complex pathogenesis, the tolerability of disease onset, and limited treatment alternatives in advanced stages.
Overall, immune cells are central to the pathogenesis of liver inflammation, fibrosis, and cirrhosis. Hepatocyte injury triggers Kupffer cells, the liver’s resident macrophages (MΦs) to release inflammatory mediators, activating other immune cells, including infiltrating MΦs, inflammatory T lymphocytes, neutrophils, and dendritic cells (DCs) [27]. These cells amplify inflammation through cytokine and chemokine production, stimulating hepatic stellate cells to transform into myofibroblasts. Myofibroblasts produce excessive extracellular matrix, leading to fibrosis and, ultimately, cirrhosis if inflammation persists. Additionally, liver sinusoidal endothelial cells play a crucial role in orchestrating the recruitment and activation of natural killer (NK) cells during acute inflammatory liver injury [28]. The complex interplay of these cells and their signaling pathways drives a cycle of injury and inflammation. Therefore, effective therapies for inflammation-mediated liver diseases must address both tissue repair and immune modulation to prevent fibrosis progression.
Therapeutic potential of MSCs in treating liver diseases
MSCs have emerged as a promising therapeutic option for treating liver diseases due to their unique biological properties, including their ability to differentiate into various cell types, modulate immune responses, and promote tissue repair. MSCs are multipotent stromal cells that can be isolated from a variety of tissues, including bone marrow (BM), adipose tissue (Ad), and birth-associated tissue like umbilical cord (UC) [11, 29]. Their therapeutic potential in liver diseases lies in their capability to treat multiple pathological processes, including inflammation, fibrosis, and hepatocyte loss, which are central to the progression of many hepatic disorders [30,31,32].
One of the key mechanisms by which MSCs exert their therapeutic effects in liver diseases is through immunomodulation (Fig. 1) [33]. Chronic liver diseases are often marked by excessive and persistent inflammation, and MSCs possess potent immunomodulatory properties. These effects are largely mediated by the secretion of bioactive molecules, including anti-inflammatory cytokines and growth factors. MSCs modulate T cells by secreting galectins (Gal) 1, 3, and 9 [34, 35], prostaglandin E2 (PGE2) [36], interleukin (IL)-10 [37], IL-11 [38], IL-25 [39], IL-1 receptor antagonist (IL-1RA) [40], nitric oxide [41], indoleamine 2, 3-dioxygenase (IDO) [42], heme oxygenase (HO)-1 [43], human leukocyte antigen G (HLA-G) [44,45,46], hepatocyte growth factor (HGF) [47], transforming growth factor beta (TGF-β) [48], programmed death-ligands (PD-L) 1 and 2 [49], or through cell-cell contact via molecules such as Jagged-1 [50], Fas ligand (FasL) [51], PD-L1 and 2 [52, 53], intercellular adhesion molecule-1, and vascular cell adhesion molecule 1 [54]. Additionally, MSCs promote regulatory T cell (Treg) induction [55] while inhibiting T helper (Th) 1 [56] and Th17 cells [57] through mitochondrial transfer. In terms of B cell modulation, MSCs secrete IL-1RA [58], PGE2 [59], IL-35 [60], IDO [61], and chemokine ligand 2 (CCL2) [62], or act through cell-cell contact via PD-L1 and 2 [52]. For NK cells, MSCs secrete PGE2, IDO [63], HLA-G [45], TGF-β [64], and also directly interact through HLA-G [65]. MSCs modulate DCs by secreting IL-6 [66], PGE2 [67], HO-1 [68], growth-regulated oncogene-γ [69], Gal-1, or through Jagged-2-mediated contact [70]. In MΦs, MSCs exert effects by secreting IL-1RA [58], CCL2 [71], TGF-β [72], PGE2 [73], tumor necrosis factor-α-stimulated gene/protein (TSG)-6 [74], stanniocalcin (STC)-1 [75] and STC-2 [76], insulin-like growth factor (IGF)-2 [77], keratinocyte growth factor (KGF) [78], and IDO [79]. Moreover, MSCs release TSG-6 to inhibit polymorphonuclear neutrophil (PMN) infiltration [74], while superoxide dismutase-3 reduces PMN respiratory burst [80]. Additionally, MSCs secrete HGF [81], PGE2 [82], CCL2 [83], and HLA-G [84] to enhance the induction of myeloid-derived suppressor cells. By creating an immunosuppressive environment, MSCs mitigate the chronic inflammation driving liver damage, thereby slowing disease progression and promoting tissue repair.
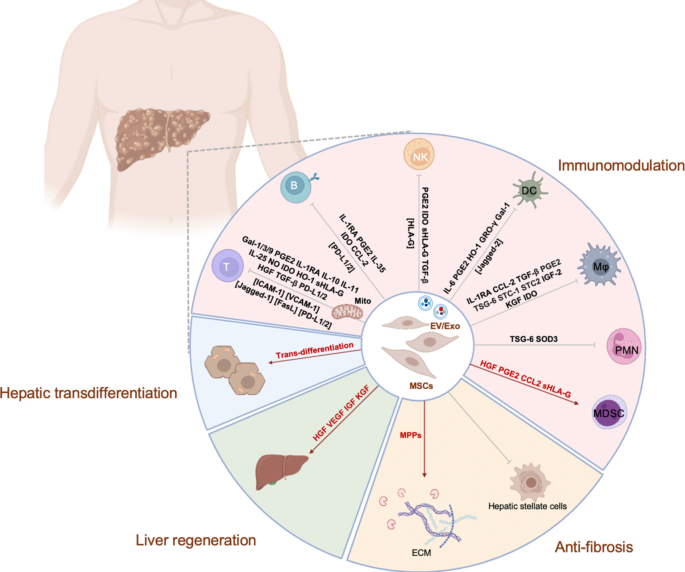
Fibrosis, a hallmark of chronic liver disease leading to cirrhosis, is another important target for MSC therapy. Fibrosis results from the excessive deposition of extracellular matrix (ECM) components, primarily collagen, produced by activated hepatic stellate cells in response to chronic liver injury. MSCs have been shown to attenuate liver fibrosis through several mechanisms (Fig. 1). Firstly, they can directly inhibit the activation of hepatic stellate cells, reducing the synthesis and deposition of ECM components [85]. Secondly, MSCs secrete matrix metalloproteinases, enzymes that degrade the ECM, thereby promoting the resolution of fibrosis [86]. Furthermore, MSCs can induce apoptosis in activated hepatic stellate cells, further contributing to the reduction of fibrotic tissue [87]. By targeting the fibrosis process, MSCs help preserve liver structure and function and may even delay or prevent the onset of cirrhosis [88, 89].
In addition to their immunomodulatory and anti-fibrotic effects, MSCs have the potential to contribute to liver regeneration (Fig. 1). MSCs can differentiate into hepatocyte-like cells under certain conditions [90, 91], providing a source of new functional hepatocytes to replace damaged or lost liver cells. Although the efficiency of MSC differentiation into fully functional hepatocytes remains an area of ongoing research, their ability to enhance liver regeneration is also supported by paracrine effects. MSCs secrete a variety of growth factors [92, 93], such as HGF, vascular endothelial growth factor, IGF, and KGF, which stimulate the proliferation of endogenous liver progenitor cells and mature hepatocytes. This regenerative capability is particularly valuable in patients with extensive hepatic injury or liver failure, whose liver functions are compromised.
Another significant aspect of MSC therapy is their low immunogenicity and relative safety profile. Unlike many other cell types, MSCs do not express high levels of major histocompatibility complex class II molecules, which allows them to evade recognition and attack by the host immune system. This property makes allogeneic MSC infusion a viable recourse, expanding the availability of MSC-based therapies to a broader patient population [94, 95].
Despite the promising results seen in preclinical studies and early clinical trials, challenges remain in optimizing MSC therapy for liver diseases. These include improving the migratory efficiency of MSCs to the liver [96], ensuring their long-term survival and function post-infusion, and understanding the mechanisms underlying their therapeutic effects in greater detail. Nevertheless, the multifaceted roles of MSCs in modulating immune responses, reducing fibrosis, and promoting liver regeneration make them a compelling therapeutic candidate for a wide range of liver diseases [97]. As research progresses, MSC-based therapies have the potential to offer new hope for patients with conditions that are currently difficult to treat.
Current clinical trials of MSC therapy for liver diseases: overview
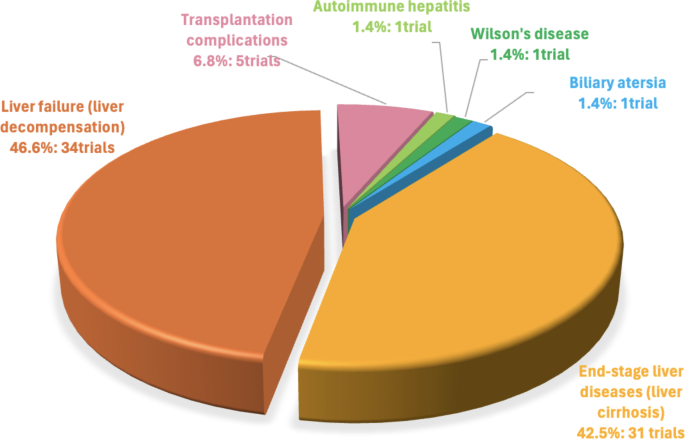
Numerous reviews have summarized ClinicalTrials.gov data on MSC therapy for liver diseases [98, 99]. However, this field is rapidly evolving. This study provides an updated overview, incorporating data from 62 trials reported in 2022 [100]. Our analysis considers key factors influencing treatment outcomes, including the source of MSCs, and the route of administration. This comprehensive approach allows for a more nuanced understanding of current MSC therapies and identifies areas for future research and development in this promising area of liver disease treatment. As of August 2024, a total of 73 clinical studies involving MSCs for treating liver disorders have been registered on NIH Clinical Trial Database (https://ClinicalTrials.gov/). The primary focus of these trials is on end-stage liver diseases or liver cirrhosis (31 trials) and liver failure or decompensation (34 trials), with fewer trials targeting other liver diseases, including transplantation complications (5 trials), and single trials for autoimmune hepatitis, Wilson’s disease, and biliary atresia (Fig. 2).
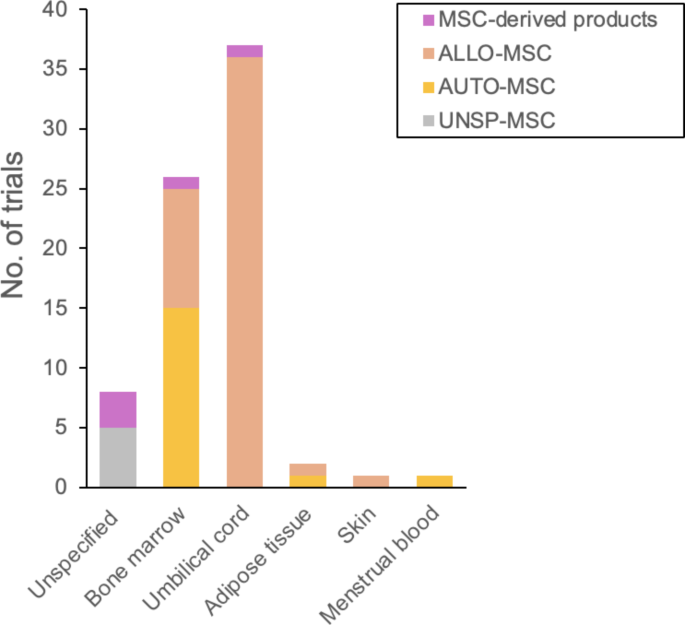
The sources of MSCs used in these studies are diverse (Fig. 3). The most common sources are UCMSCs (35 trials) including one exosome trial, and BMMSCs (24 trials) including one trial using BMMSC-derived hepatocytes. Additionally, two trials use both UCMSCs and BMMSCs. AdMSCs were utilized in 2 trials, whereas skin tissue MSCs and menstrual blood MSCs were each used in 1 trial. Seven trials involve MSCs without specifying the donor or tissue source, including 2 trials that explore MSC-derived extracellular vesicles (EVs). One trial uses autologous MSC-derived hepatocyte progenitors from an unspecified tissue source. Among the 37 UCMSC trials, all are considered allogeneic. Allogeneic BMMSCs are used in 10 trials, while allogeneic AdMSCs and skin tissue MSCs are each used in 1 trial. Autologous MSCs are employed in 19 trials, primarily from BMMSCs (16 trials), with one trial using autologous AdMSCs and another one using autologous menstrual blood MSCs.
The majority of these trials are in early phases, with 16 phase 1 trials, 28 combined phase 1/2 trials, 15 phase 2 trials, 1 combined phase 2/3 trial, 2 phase 3 trials, 1 phase 4 trial; 10 trials are unspecified (Table 1). A significant number of participants are involved across these phases, totaling 6,013, with 261 in phase 1 trials, 2,121 in combined phase 1/2 trials, 1,673 in phase 2 trials, 66 in the combined phase 2/3 trial, 730 in phase 3 trials, 5 participants in the phase 4 trial; 1,157 participants are unspecified (Table 2). These trials highlight a strong interest in the immunomodulatory and regenerative potential of MSCs, particularly in the use of allogeneic sources.
Current clinical trials of MSC therapy for liver diseases
Specific chronic liver disease
MSC therapy is being explored in clinical trials for its potential to treat various chronic liver diseases, including Wilson’s disease, autoimmune hepatitis, and biliary atresia, with specific dosing strategies tailored to each condition (Table 3). In patients with Wilson’s disease-related liver injury or cirrhosis, MSCs derived from healthy donor BM are infused into patients at a dose of 1 million cells per kg of body weight, with 50 million cells delivered via the hepatic artery and the remaining cells via a peripheral vein. This approach aims to promote liver regeneration, thereby reducing fibrosis, decreasing the need for chelating agents, and potentially delaying or avoiding liver transplantation. In autoimmune hepatitis, an immune-mediated liver disease traditionally treated with corticosteroids and/or immunosuppressants, a clinical trial is testing UCMSCs. Participants are randomly assigned to receive either conventional treatment combined with UCMSC infusions with 1 million cells per kg of body weight intravenously every four weeks for 12 weeks, or conventional treatment with a placebo. The study monitors liver enzyme levels, histology, and relapse rates to assess safety and efficacy. Biliary atresia, the leading cause of pediatric liver transplantation, is being addressed in another trial where Kasai-operated patients receive two UCMSC transplants via the hepatic artery, with a dosage of 1 million MSCs per kg of body weight per transplant. Safety is evaluated by monitoring adverse events post-injection, while efficacy is measured using the Pediatric End-Stage Liver Disease (PELD) score, liver function tests, and liver biopsy results. These trials demonstrate the scope of MSC therapy, with specific dosages and delivering strategies, to address the complex challenges of chronic liver diseases, offering new, regenerative treatment options.
End-stage liver disease (liver cirrhosis)
Clinical trials of MSC therapy for end-stage liver disease (liver cirrhosis)
Cirrhosis is a severe condition resulting from prolonged liver damage caused by alcohol or other toxins, viral hepatitis, autoimmune hepatitis, metabolic diseases, and certain medications, to name a few. This damage leads to progressive scarring, which gradually replaces normal liver cells, causing distortion of the parenchyma architecture and the formation of regenerative nodules. Liver cirrhosis represents a late stage of hepatic fibrosis and often progresses to liver failure. Currently, liver transplantation is one of the few effective treatments available for cirrhosis, but it is associated with significant challenges, including a shortage of donors, surgical complications, graft rejection, and high costs. To address these challenges, MSC therapy has emerged as an optional treatment. There are currently 31 clinical trials investigating MSC therapy for cirrhosis (Table 4). One trial involves autologous AdMSC therapy for Hepatitis C virus (HCV)-related cirrhosis, where one million cells per kg are injected via the peripheral vein and three million cells per kg are administered into the right hepatic artery. Another trial explores allogeneic BMMSC therapy for Hepatitis B virus (HBV)-related cirrhosis, with cells delivered via the portal vein or hepatic artery, alongside standard antiviral treatment. A third trial involves intravenous infusion of allogeneic BMMSCs for primary biliary cholangitis, a biliary-specific inflammatory disease that leads to liver cirrhosis and eventually failure. Additionally, nine clinical trials are using autologous BMMSCs, and 12 trials are using UCMSCs, typically administered at a dose of approximately one million cells per kg via peripheral vein or hepatic artery. One trial combines autologous BMMSC therapy with allogeneic UCMSCs administered weekly for six weeks. One trial uses autologous MSCs harvested from menstrual blood at a dose of one million cells per kg via intravenous infusions twice per week for two weeks, in combination with conventional treatment like antiviral drugs. Three trials use MSCs from unspecified donation and tissue sources with one of these combining regulatory T cell transplantation, while one trial employs autologous BMMSC-derived hepatocytes and another one uses unspecified MSC-derived hepatocyte progenitors. In most trials, liver function is monitored by analyzing serum levels of alanine aminotransferase (ALT), total bilirubin, prothrombin time, prealbumin and albumin from pre-transfusion and post-transfusion. Improvements are also evaluated using the Model for End-Stage Liver Disease (MELD) score, the Child-Pugh score, or assessment of patients’ quality of life.
Results of clinical trials evaluating MSC therapy for end-stage liver disease (liver cirrhosis)
For end-stage liver diseases or liver cirrhosis, two clinical trials have published results. In a trial registered under NCT01741090, twelve patients (11 males, 1 female) with biopsy-proven alcoholic cirrhosis, who had been abstinent from alcohol for at least six months, were enrolled to evaluate the efficacy of autologous BMMSCs [101]. BMMSCs were isolated from BM of each patient, cultured for one month, and then administered at a dose of fifty million cells twice at weeks 4 and 8 via the hepatic artery. One patient was withdrawn from the trial due to alcohol consumption, leaving 11 patients who completed follow-up biopsies and laboratory tests 12 weeks after the second injection. The primary outcome was the improvement of liver histology in 6 patients (54.5%) following BMMSC therapy according to the Laennec fibrosis scoring system. Regarding the secondary outcome, 10 patients of the 11 patients (90.9%) experienced an improvement in liver function, as indicated by their Child-Pugh scores following BMMSC therapy. Additionally, significant reductions were observed in levels of transforming growth factor-β1, type 1 collagen, and α-smooth muscle actin following BMMSC therapy. The study also reported no significant complications or side effects. In another trial registered under NCT01729221, forty patients with HCV-related liver cirrhosis were randomized into the control group with standard liver-supportive treatment and the MSC-treated group, each with 20 patients [102]. In the MSC-treated group, patients received granulocyte colony-stimulating factor for 5 days followed by autologous BMMSC infusion via the peripheral vein. Among the MSC-infused patients, 54% showed near normalization of liver enzymes and improvement in liver synthetic function. Significant changes were reported in albumin, bilirubin, prothrombin concentration, and ALT levels. Therefore, autologous BMMSC therapy in patients with alcoholic cirrhosis or end-stage liver disease demonstrated histological and quantitative improvements in liver synthetic functions and hepatic fibrosis.
Liver failure (liver decompensation)
Clinical trials of MSC therapy for liver failure (liver decompensation)
The leading cause of death in patients with cirrhosis is the development of acute-on chronic liver failure (ACLF), a syndrome associated with high mortality. Currently, liver transplantation is the only effective treatment for ACLF or decompensated liver diseases, but the availability of donor organs is severely limited. Other treatments, including artificial liver support systems, have not shown an improvement in survival rates. ACLF is characterized by an increased systemic inflammatory state, coupled with impaired liver regeneration, leading to multiorgan failure. MSC therapy is a promising strategy for treating ACLF, as well as chronic or acute liver failure, due to the immunomodulatory and regenerative properties of these cells. To date, 34 clinical trials are investigating MSC-based therapies, including two utilizing cell-free products, for the treatment of liver failure or liver decompensation (Table 5). Among these, five trials involve autologous BMMSC infusion. Two trials focus on treating decompensated liver through peripheral blood injection, with one of them involving a one-time infusion of 300 million cells. Another trial targets HBV-related liver failure, a critical condition of extensive liver cell necrosis, with follow-up monitoring for the incidence of hepatocellular carcinoma and mortality. One trial is exploring the use of portal vein infusion combined with Pioglitazone, which has shown benefits in ameliorating liver dysfunction in type 2 diabetic (T2D) patients with non-alcoholic fatty liver disease. Additionally, another trial focuses on decompensated cirrhosis by co-infusing autologous hematopoietic stem cells (HSCs) and BMMSCs via the hepatic artery, as autologous HSCs have demonstrated the capability to improve liver function. In addition to autologous therapies, 27 trials are investigating the use of allogeneic MSC sources. These include 18 trials using UCMSCs, 3 using BMMSCs, 1 combining BMMSCs and UCMSCs, 1 using AdMSCs, 1 using skin-derived MSCs, and 3 using unspecified sources. For instance, allogeneic AdMSCs (Stemchymal®) are being tested for acute liver failure (ALF) and ACLF at doses of 0.5 million versus 2 million cells per kg via intravenous injection. Allogeneic skin-derived ABCB5-postive MSCs are being used to treat ACLF at three doses of 2 million cells per kg via intravenous injection on days 0, 5, 13. Among the three trials with allogeneic BMMSCs, one trial focuses on ACLF with 4 doses of 2 million cells per kg intravenously administered on days 1, 4, 11, and 18. Another trial addresses HBV-related liver failure with doses of 0.5, 2 or 5 million cells per kg via intravenous injection, while the third trial administers BMMSCs via the portal vein or hepatic artery in conjunction with conventional therapy. Allogeneic UCMSCs are the most commonly used in treating liver failure or liver decompensation, including HBV or alcohol-related AFLD, with doses ranging from 10 to 100 million cells via intravenous administration. Additionally, three trials using allogeneic MSCs of unspecified sources are being conducted in patients with AFLD, with treatments involving 3 or 4 doses of approximately 1 million cells per kg via peripheral vein infusion. Moreover, one trial compares the effects of allogeneic BMMSCs and UCMSCs in treating HBV-related liver failure. This trial administers weekly intravenous infusions of 0.1, 1, or 10 million cells per kg for 8 weeks. For the two cell-free therapies, one trial uses EVs for treating ALF and ACLF, with a weekly injection of 1010 MSC-EV particles per 100 ml for four weeks. The other trial uses UCMSC-derived exosomes for treating decompensated liver cirrhosis, with a final dose of 40 mg over three weeks. These trials typically assess liver function, as well as survival rate.
Results of clinical trials evaluating MSC therapy for liver failure (liver decompensation)
Among the clinical trials for liver failure or liver decompensation, five have published results. In a trial registered under NCT01322906, the efficacy of allogeneic BMMSCs was evaluated in 110 patients with HBV-mediated ACLF [103]. Patients were randomized into two groups: 56 received weekly infusions of 0.1-1 million cells/kg for 4 weeks, while 54 received standard medical therapy (SMT). The BMMSC group had a 24-week survival rate of 73.2% (95% CI: 61.6-84.8%) compared to 55.6% (95% CI: 42.3-68.9%) in the SMT group (P = 0.026). In another trial registered under NCT00476060, the safety of autologous BMMSCs was assessed in 53 patients [104]. A control group of 105 patients was matched for age, sex, and biochemical indices, including ALT, albumin, total bilirubin, prothrombin time, and MELD. At 192 weeks of follow-up, there were no significant differences in the incidence of hepatocellular carcinoma or mortality between the two groups. This indicates that autologous BMMSC transplantation is safe for patients with liver failure due to chronic hepatitis B. In the trial registered under NCT04243681 assessing the safety of infusing a combination of autologous HSCs and MSCs in patients with decompensated liver cirrhosis, the cells were infused through hepatic artery [105]. The study concluded that the combination of autologous HSC and MSC infusion is a safe procedure for patients with decompensated liver cirrhosis, with further evaluation in larger studies currently ongoing. Another trial registered under NCT05227846 using UCMSCs administered a single injection of UCMSCs at predetermined doses (ranging from 5 × 107 to 2 × 108) following the ‘3 + 3’ rule [106]. The primary outcomes measured were the incidence of adverse events and changes in MELD scores from baseline to the 28th day. Secondary outcomes included changes in the MELD score over time, the incidence of complications associated with decompensated cirrhosis, liver transplant-free survival, and the occurrence of liver failure. Patients in this ongoing study will be followed up to further evaluate safety and tolerability of UCMSC treatment in patients with decompensated liver cirrhosis. In the other trial registered under NCT01724398, a total of 110 patients with HBV-ACLF were enrolled and assigned to one of four treatment groups: control (n = 30), UCMSC (n = 30), plasma exchange (PE) (n = 30), and UCMSC + PE (n = 20) [107]. The endpoints were the need for liver transplantation or death. While both the UCMSCs alone and the combination of UCMSCs with PE demonstrated good safety, the UCMSC group showed similar outcomes to the control and PE groups. In contrast, the UCMSC + PE group exhibited the lowest rates of liver transplantation and mortality at 30, 60, and 90 days post-treatment compared to the other groups; however, these differences did not reach statistical significance. Though further studies are needed, with over thirty trials underway, MSC therapy offers an encouraging alternative to liver failure or decompensation, particularly those of autologous and umbilical cord-derived sources.
Transplantation complications
Liver transplantation is used to restore liver function in end-stage liver diseases or liver failure; however, its complications often lead to lethality. To date, five allogenic MSC-based trials have been conducted, including two using BMMSCs, two using UCMSCs, and one using EVs to treat or prevent complications associated with liver transplantation (Table 6). The introduction of calcineurin-based immunosuppression has significantly improved patient and graft survival in pediatric liver transplantation. However, calcineurin inhibitor (CNI) toxicity reduces the quality of life for many recipients and results in significant morbidity. Moreover, CNI is unable to prevent long-term allograft inflammation and fibrosis. MSCs are believed to have potent immunomodulatory properties, potentially promoting allograft tolerance and reducing the toxicity from high-dose CNI exposure. In one trial, allograft BMMSCs were administered in two doses of one million cells, first via the portal vein and then through the peripheral vein, to evaluate their safety in pediatric living-donor liver transplantation and their effect on immunomodulation and graft survival. Despite the benefits of current immunosuppressive agents in reducing acute cellular rejection after liver transplantation, the rate remains high, reaching 20–50%. Furthermore, the long-term side effects of these treatments pose a major challenge for liver transplant recipients, increasingly being recognized as an unmet clinical need. One trial assesses the safety and tolerability of third-part BMMSC administration with a dose of 1.5 to 3 million cells per kg after liver or kidney organ transplantation. One trial is focused on assessing the impact of UCMSCs on improving conditions in liver transplant patients. In this trial, UCMSCs are administered in conjunction with 12 weeks of standard immunosuppressive agents to evaluate their safety. Another trial is investigating UCMSCs’ effectiveness in treating or preventing ischemic-type biliary lesions, a significant cause of graft loss and mortality after orthotopic liver transplantation. Patients in this trial receive UCMSCs at a dose of one million cells per kg once a week for the first month, and then once a month for six months (a total of 9 doses). In addition to BMMSC or UCMSC therapies using intravenous infusion, another trial uses MSC-derived EV from unspecified sources with a dose of 1010 particles per 100 ml for ACLF after liver transplantation. Among these trials, one BMMSC trial registered under NCT01429038 has published results, showing no side effects from MSC infusion at day 3 after liver transplantation, though the infusion did not promote tolerance [108]. This study showed no toxicity after a single MSC infusion, but was insufficient to allow the withdrawal of immunosuppression. It opens the path for further trials with repeated infusions or combining MSCs with other immunosuppressive cells, such as Tregs, in liver transplant recipients. Overall, the five allogeneic MSC-based trials, including two BMMSCs, two UCMSCs, and one EV trial, target complications in liver transplantation. Additionally, results from a clinical trial registered as ChiCTR2000037732 on another platform chictr.org.tw indicated that allogeneic UCMSCs are comparable to rituximab treatment for the prophylaxis of antibody-mediated rejection in ABO-incompatible liver transplantation [109]. These studies evaluate immunomodulatory properties, safety, and graft tolerance, with ongoing research needed to address remaining challenges.
Limitations and challenges
While MSC-based treatments for liver diseases have shown promise in clinical practice, several limitations and challenges remain. One significant issue is the low engraftment and retention rates of MSCs in the liver following intravenous infusion [110], which can reduce their therapeutic efficacy. Additionally, the variability in MSC sources, methods of isolation, and culture conditions may lead to inconsistent quality and functionality of the cells, complicating standardization across studies [111]. Immunogenicity is another concern, as MSCs may elicit variable immune responses in recipients, potentially resulting in adverse outcomes [112]. Furthermore, our understanding of the detailed mechanisms through which MSCs exert their effects is still limited, hindering the optimization of treatment protocols and patient selection criteria. Addressing these challenges is essential for advancing MSC therapies in the clinical management of liver diseases.
Summary
In our study, we reviewed the current clinical trials of MSC therapy for treating various liver diseases. Chronic liver diseases often progress to cirrhosis and liver failure, for which treatment options are limited. MSCs, with their immunomodulatory and regenerative properties, as well as their ability to transdifferentiate into hepatocytes, offer promising alternatives. Based on current clinical trials, According to current clinical trials, UCMSCs are the most commonly used source, offering an off-the-shelf solution. Autologous MSC-derived hepatocytes specifically target cirrhosis, while allogeneic sources are used to address complications in liver transplantation. Current clinical results indicate that MSC therapy is both safe and effective. However, further large-scale studies are necessary to confirm its broader applications.
This article is based on research initially published in Stem Cell Research & Therapy, © The Author(s) 2024, available under the Creative Commons Attribution 4.0 License.
